Kaiser Permanente researchers part of multi-system, CDC analysis
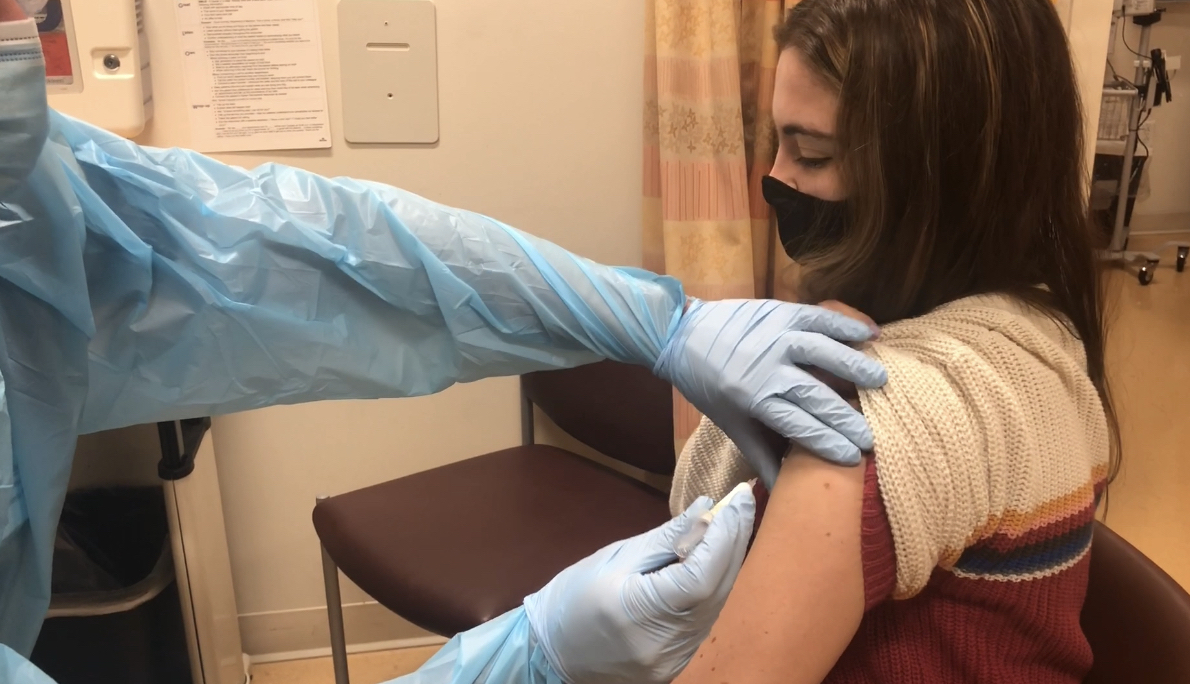
An analysis of early data on the effectiveness of the Pfizer/BioNTech COVID-19 vaccine in children and teens found that a third shot, or booster, extended protection against emergency department (ED) and urgent care visits in 16- and 17-year-olds. The data also reflected a significant decline in effectiveness of the vaccine against the omicron variant compared with the earlier delta variant.
The study was published March 1 in the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report.

“These results, while early, suggest a real benefit for a third, booster vaccination in teens that may extend protection against disease severe enough to seek immediate care,” said Nicola Klein, MD, PhD, lead author of the study and director of the Kaiser Permanente Vaccine Study Center, part of the Kaiser Permanente Division of Research.
It is one of the first studies to provide insights into the likely effectiveness of the Pfizer/BioNTech vaccine in young people during the time of the omicron variant, and the findings show differences in how the virus behaved between the periods when delta and omicron were dominant.
In 12- to 17-year-olds, effectiveness against ED and urgent care visits was lower during omicron than delta, offering no significant protection 5 months after the second dose.
But the third (booster) dose had an impact. For those aged 12-17, over the time period including both variants, effectiveness of the vaccine ranged from 76% to 83% during the first few months after the second dose, and declined to 38% to 46% at 5 months. For teens aged 16-17, a third (booster) shot increased effectiveness to 86% for the week after the inoculation.
Effectiveness against hospitalization for adolescents aged 12-17 ranged from 92% to 94% in the 14 to 149 days after the second dose, and 73% to 88% at 150 days.
The study examined children and teens who were hospitalized or went to an emergency department or urgent care facility at 8 health systems for symptoms of COVID-19. It included 1,699 children aged 5 to 17 hospitalized for a COVID-19-like illness, and 39,217 visits to emergency departments and urgent care facilities.
The research, supported by the CDC, was one of a series of vaccine effectiveness studies carried out by the VISION network of 8 health systems, including Kaiser Permanente Northern California.
New data on younger children
This study provided some of the first insights into vaccine effectiveness for younger children since the initial clinical trials for the vaccine, though the total number of children in the study was limited because relatively few younger patients were seen in hospitals, EDs, and urgent care facilities, and because the Pfizer-BioNTech vaccine was authorized for those under 12 later than other age groups.
In children 5-11, the analysis calculated vaccine effectiveness after the second dose of 46% against ED and urgent care visits, and 74% against hospitalization with COVID-19. However, the calculation on hospitalization was based on a small number of cases, so did not reach statistical significance.
“These data are early and not definitive, but they are promising that vaccination in 5-to-11-year-olds will be protective against hospitalization,” Klein said.
The study authors said the findings in the 5-17 age group were consistent with previous studies showing high effectiveness of the Pfizer-BioNTech vaccine against severe outcomes in adults, and that also showed a decline in effectiveness after the second dose in adolescents and adults and during the Omicron period.
The VISION network partners continue to collect information about vaccine effectiveness in all ages. Additional time and data will be particularly important for understanding how well vaccines are working in in children as well as adolescents, Klein said, as analyses will be strengthened with increasing time since vaccination and larger numbers of health care encounters among patients of all ages.
Additional Kaiser Permanente Division of Research co-authors were Ned Lewis, MPH, Kristin Goddard, MPH, Bruce Fireman, MA, and Ousseny Zerbo, PhD.
# # #
About the Kaiser Permanente Division of Research
The Kaiser Permanente Division of Research conducts, publishes and disseminates epidemiologic and health services research to improve the health and medical care of Kaiser Permanente members and society at large. It seeks to understand the determinants of illness and well-being, and to improve the quality and cost-effectiveness of health care. Currently, DOR’s 600-plus staff is working on more than 450 epidemiological and health services research projects. For more information, visit divisionofresearch.kaiserpermanente.org or follow us @KPDOR.



This Post Has 0 Comments